

All the colors of the Absorption Spectra do make it kind of confusing. And these are being absorbed (with emphasis on blue). Actually, if you just burned hydrogen and looked at its spectra, you would get the Emission Spectra and not the Absorption Spectra, and this Emission Spectra would only show the bunch of blue lines, one purple line, and one red line.
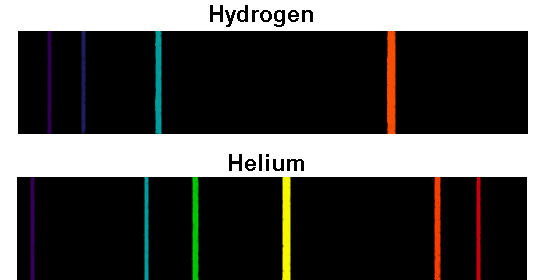
All the other colors shown are just part of the natural light being shown down on the element. This is the color that will be the opposite of the flame color on the color wheel. Remember, always look at the color area on the rainbow that is blacked out the most. So if blue is being absorbed, the opposite color would be transmitted and this color is orange. However, there are MORE dark lines in the blue region. The line spectra of excited hydrogen, neon, and argon atoms the photon wavelength and frequency. If you look at the lines for hydrogen blue, purple, and red are being absorbed. Across the top is a scale that begins Figure: Line Spectra. Therefore, all the other colors would be absorbed. Understand the processes of absorption and emission. Know that radiation of short wavelength is toxic. Understand that ionizing radiation can knock electrons off DNA. (This would be orange.) The element hydrogen turns orange when being burned and this color is transmitted to us. Learning Objectives Differentiate between a continuous and discontinuous spectrum Classify all types of radiation as being ionizing or nonionizing. This means that if there is a big dark band where blue would be, then the opposite color to blue on the color wheel is being transmitted. The plasma is sustained and maintained by inductive coupling from. The plasma is a high temperature source of ionised source gas (often argon). You are supposed to look at the dark areas of the absorption spectra and those dark areas indicate that the color which would be there is being absorbed. It is a type of emission spectroscopy that uses the inductively coupled plasma to produce excited atoms and ions that emit electromagnetic radiation at wavelengths characteristic of a particular element. To understand that the spectral emission lines of an element have the same frequencies as the lines of the absorption spectrum.I think both the absorption and emission lines are showing which colors are being absorbed.To characterize an atom by its light spectrum and to introduce spectroscopy.To distinguish between absorption spectra and emission spectra.CRC Handbook of Chemistry and Physics NSRDS-NBS 68 (1980).Ĭlick on an element to select it. Source for the values of spectral lines: CDS Strasbourg University ( link) from Reader J., and Corliss Ch.H. For this reason, a gas composed of a single atom can absorb or emit a limited number of frequencies.įor a given element, the emission spectrum (upper part of the animation) has the same frequency as its absorption spectrum (bottom part). It is "quantized" (see animation line spectrum of the hydrogen atom). One of the great discoveries of quantum mechanics is that the energy of an atom can only have certain well-defined values. The spectrum obtained can be continuous or discrete ("line spectrum"). A prism (or an array) is used to break a beam of light according to its different frequencies.


 0 kommentar(er)
0 kommentar(er)
